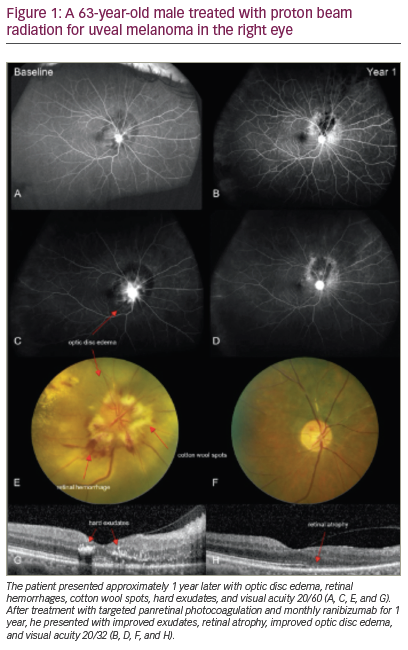
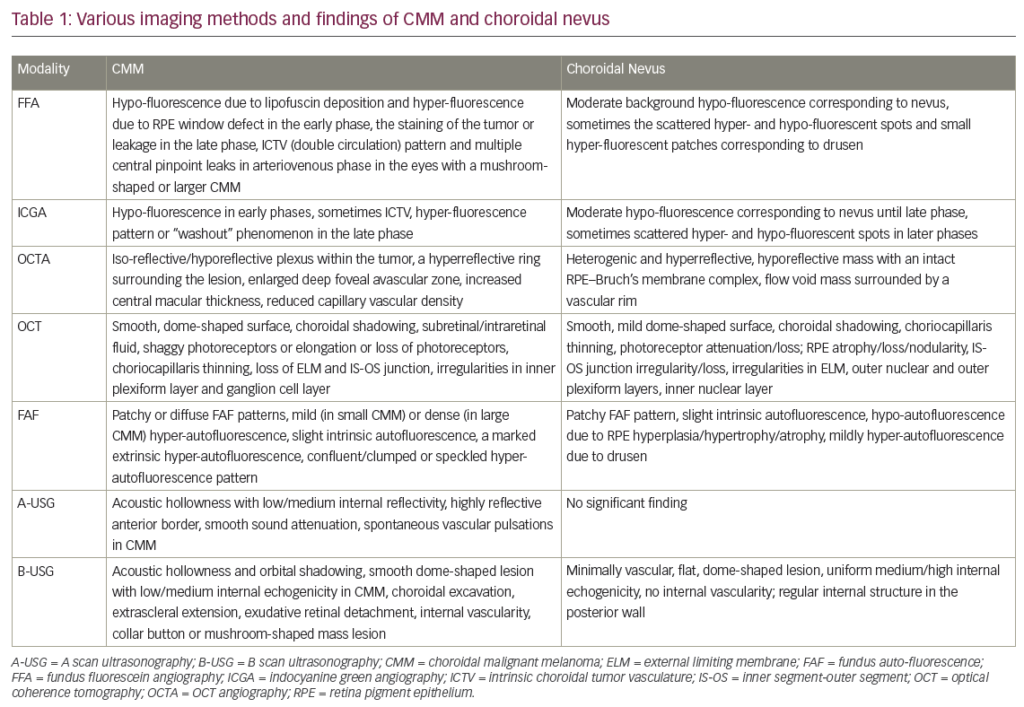
Since the first description of ‘epitheliomas’ by von Graefe in 1860, many papers have described squamous epithelial neoplasia of the surface of the eye. After several attempts were made to classify the varying presentations of these lesions, the term ocular surface squamous neoplasia (OSSN) was introduced in 19951 as a way to describe all squamous neoplastic lesions of the conjunctiva and cornea. This includes benign, noninvasive papillomas, pseudoepitheliomatous hyperplasia, and benign hereditary intraepithelial dyskeratosis, as well as conjunctival-corneal intra-epithelial neoplasia (CCIN), squamous cell carcinoma (SCC), and mucoepidermoid carcinoma.2 The American Joint Committee on Cancer Classification (AJCC) established a TMN system for classifying OSSN,3 which has been useful in standardizing the reporting of various treatment options.
While relatively uncommon, OSSN is the most common tumor of the ocular surface, with the incidence reported from 0.3 per million per year in the US4 to as high as 1.9 per 100,000 in Australia.5 A recent study from the National Institutes of Health (NIH)-AARP cohort found that the incidence of SCC of the conjunctiva was 8.4 per million and that these made up 22 % of all eye cancers in the group they studied.6 It has been demonstrated that the incidence of OSSN increases with proximity to the equator and that they are more common in lighter-skinned males. The incidence also increases with age with an average age of 56 at presentation;5 in younger patients the presence of OSSN is often associated with another underlying disorder such as the genetic defect in xeroderma pigmentosum7 and immunosuppression as is seen in patients infected with HIV.8
Like other squamous neoplasias, OSSN is thought to be the result of abnormal epithelial cell proliferation and maturation caused by underlying mutagenic factors.9 The most consistent factor associated with the development of OSSN is ultraviolet (UV) radiation.10 The influence of UV on carcinogenesis is the best explanation for the increased incidence closer to the equator and in lighter-skinned individuals. UV radiation affects p53, which has been shown to be abnormal in several patients studied with OSSN.10 Protein derived from the E6 region of the human papillomavirus (HPV) genome also complexes with the protein associated with the p53 gene. Attempts have been made to link infection with HPV to the development of OSSN, since it has been well-described as carcinogenic in other forms of SCCs, especially cervical. HPV was found in 33 % of OSSN specimens in one study, while similar studies found the incidence of HPV in conjunctival papillomas to be higher than 80 %. A recent review of the link between HPV and OSSN concluded that there was not enough evidence to say a strong association exists.11 Other reported risk factors for the development of OSSN include smoking, ocular surface injury, chemicals (petroleum products, beryllium, trifluridine, arsenic), and vitamin A deficiency.2
Treatment
Historically, OSSN has been treated surgically. Early case series reported recurrence rates of 15 % to 52 %.1 The Shields group reported a ‘notouch’ method of larger excised margins (2 mm on cornea; 4–5 mm on conjunctiva) with absolute alcohol and adjuvant cryotherapy in 1997, with lower recurrence rates.12 The challenges of complete surgical excision in patients with extensive tumors, in addition to the high recurrence rates in some series, led to a search for nonsurgical treatment modalities. In addition to avoiding surgery, topical agents treat clinically unapparent disease. Due to the theoretic advantages of topical chemotherapy, multiple agents have been used in both the primary and adjuvant treatment of OSSN.
Mitomycin C
Mitomycin C (MMC) is an antibiotic with antitumor activity that is isolated from Streptomyces spp. It functions as an alkylating agent leading to the cross-linking of DNA, thereby inhibiting DNA synthesis.13 Initially it was used as an intravenous treatment of many different solid organ tumors, but it has also been found to be effective as a topical agent. It was first reported as a therapy for corneal intraepithelial neoplasia in 1994 by Frucht-Pery in a series of three patients with central disease. They reported successful eradication without recurrence during follow up in three out of three cases with adverse reactions including hyperemia, pain, and blepharospasm.14 Further studies have demonstrated the successful treatment of OSSN, including recurrent and extensive lesions.15–20 Dosing regimens range from 0.002–0.04 % given three to four times daily, often in alternating cycles of 1 week of therapy followed by 1 week of recovery.1–3 A report of 91 eyes with OSSN, including 73 primary and eight recurrent localized CCINs treated with primary excision±cryotherapy and adjuvant topical MMC showed a recurrence rate of 0 % over an average follow up of nearly 60 months. Diffuse CCIN in 10 patients treated with primary MMC showed recurrence in two and persistent disease in one patient.21
MMC is better tolerated with treatment times of less than 2 weeks. Reported side effects include hyperemia, blepharospasm, ocular pain, punctate keratopathy, punctal stenosis, and limbal stem cell deficiency.22 Minor side effects generally resolve with cessation of therapy and/or initiation of topical steroids. Systemic side effects have not been reported. Punctal stenosis may be prevented by the use of punctal plugs prior to the initiation of therapy. In a 10-year review of MMC treatment, short-term complications were found to have occurred in 52 % of patients but were severe enough to necessitate cessation of therapy in only 7 %.23 In the same series of patients, the risk for developing limbal stem-cell deficiency after treatment with MMC was reported to be 12 %.23 It is recommended to allow for complete healing of conjunctival/corneal tissues after biopsy or excision prior to initiating MMC topical therapy to reduce the risk for corneo-scleral melt. Confocal analysis of the corneal endothelium in eyes treated with MMC for OSSN demonstrated no significant change in cell count after therapy.24
Despite the lack of randomized studies involving MMC treatment of OSSN there is sufficient evidence to suggest efficacy as both an adjuvant and primary therapy, although the risk for limbal stem cell deficiency may be unacceptably high in patients that could potentially be treated with other modalities.
5-Fluorouracil
5-fluorouracil (5-FU) is a pyrimidine analog that was first synthesized in 1957. It has been shown to function as an antitumor medication via several mechanisms. First, it acts as an inhibitor of thymidylate synthetase, which prevents incorporation of thymidine into DNA during the S-phase of cell division. It can also be incorporated into RNA, inhibiting RNA and therefore protein synthesis, and it has been shown to affect the actin cytoskeleton.25 5-FU was first reported as a topical treatment for premalignant ocular surface lesions in 1986 by de Keizer et al. who saw an improvement in conjunctival and corneal actinic keratoses after a patient had been treated topically for periorbital actinic keratoses.26 They reported successful treatment in three of five patients with no reported side effects. Yeatts et al. published a case series in 1995 of three patients who received 5-FU as adjuvant therapy following excision and three who were treated with 5-FU as the initial therapy. They reported that four of the six patients had complete response with prolonged disease-free follow up.27 5-FU has been traditionally dosed in a 1 % artificial tear-based solution four times per day for up to 4-week treatment cycles. Yeatts described an alternate treatment course involving 2–4 day pulsed therapy with 30–45 days between cycles. In this study, five of the seven patients had resolution of the OSSN with only 5-FU treatment and three out of those five required only one cycle of treatment. The outcomes from this alternative treatment course were similar to the longer treatment regimen with an improvement in side effects.28 A series of 41 consecutive OSSN cases treated with 4-week cycles of 5-FU, including 22 who received 5-FU as the primary therapy, showed complete regression in all patients with an average follow up of almost 90 months. Three patients in the primary 5-FU group had early recurrence, but all three showed regression with additional cycles of treatment.29 A case report from Japan showed successful treatment of CIN in a patient by changing from MMC to 5-FU, which suggests the possibility of utilizing 5-FU as a treatment for OSSN refractory to MMC therapy.30 Conversely, it has also been reported that 5-FU treatment failures can respond to MMC.28
Side effects attributed to 5-FU topical therapy for OSSN are similar to those of MMC. They commonly include conjunctival injection, keratopathy, ocular pain, and skin irritation.22 As previously mentioned, in the patients who underwent short pulses of therapy, these side effects were minimized.28 Topical steroids have been reported to improve patient tolerance if they experience side effects. Lacrimal drainage stenosis, seen with systemic 5-FU therapy, has not been reported with topical treatment. Parrozzani et al. showed that 5-FU appears to selectively destroy neoplastic cells using full-thickness confocal microscopy. They found no significant differences between treated and the nontreated control eyes in a quantitative analysis of corneal endothelium, epithelium, stroma, and sub-basal nerve plexus suggesting the long-term safety of 5-FU therapy.29
Interferon
Interferon alpha-2B (IFNα-2B) is a recombinant form of a human type I interferon. Interferons are natural glycoproteins that are released by host cells as a response to the presence of pathogens to upregulate the immune response. They are named for their ability to ‘interfere’ with viral replication and have been used as a treatment in some human viral infections such as hepatitis B and C. They have also been found to have potent anti-tumor capabilities and are used in hairy cell leukemia, Kaposi sarcoma, various skin cancers, and SCC of the cervix.31 They are known to activate many signaling cascades including the JAK and MAP kinase pathways as well as others involved with protein transcription. Some studies indicate that the p38 MAP kinase pathway may play a role in the anti-tumor properties of interferons.32 Studies in skin cancershow a downregulation of interleukin (IL)-10, an immunosuppressive cytokine found in some skin cancers,33 and an upregulation of IL-2 and IFN-gamma, which aid in T-cell response.34 Interferons have also been shown to modulate the regulation of oncogenes, including p53.35
In 1994, Maskin first reported regression of limbal epithelial dysplasia with administration of topical interferon.36 Many other reports have shown that IFNα-2B can be effective as primary and adjuvant therapy of OSSN, both topically and intralesionally.31,37–39 Several large case series recently reported by Shah40 and Shields41 have shown the effectiveness of IFNα-2B, utilizing outcomes measured by the AJCC . Shah et al. found that topical IFNα-2B had an 83 % success rate in complete control of OSSN in 23 tumors. Recurrence was noted in one case and tumors in new locations occurred in two cases. The Shields paper reported 80 patients who were treated with a combination of topical and/or injection of IFNα-2B combined with surgery when necessary. They reported an overall response rate of 95 % with 5 % recurrences over 1 year of follow up. The least responsive classification was Tis (carcinoma in situ). Dosing is typically 1 million IU/mL four times per day, and is usually given until complete clinical resolution has been achieved, though the necessary treatment time past response has not been established. Galor et al. looked at a comparison between the 1 million IU/mL dose and a higher dose (3 million IU/mL) and found no significant difference in the response rates.42 Topical IFNα-2B has retrospectively been compared to the gold standard of surgical excision by Sturges et al. In 15 patients who received IFNα-2B, two required surgical excision for a lack of response, but there were no recurrences in 36 months of follow up. Of the 14 patients who underwent primary surgical excision using the Shields technique there were no recurrences.43
One of the likely reasons for the increase in IFNα-2B use is the relative paucity of adverse effects. In the Shields series, side effects included hyperemia, irritation, follicular conjunctivitis, and superficial punctate keratitis, which occurred in less than 5 % of the patients.41 In the US, topical IFNα-2B is a more expensive therapy than 5-FU and MMC, but with the decreased incidence and severity of adverse effects, increased patient tolerance, and acceptable efficacy it is a viable topical option for the treatment of OSSN.
Other Potential Therapies
Other nonsurgical treatments may be viable options as well. One that has recently been reported is anti-vascular endothelial growth factor (VEGF) therapy. Antibodies against VEGF, a pro-angiogenic signal, were originally developed as an antitumor medication and have been used to treat colon, rectal, breast, pancreatic, renal cell, and non-small cell lung cancers. One of these antibodies, bevacizumab, has been used with some success as a treatment for SCCs in the head and neck region44 as well as recurrent cervical tumors.45 Animal studies have shown that in some models of OSSN tumors there is a dramatic upregulation of both VEGF-A and VEGF-R1T.46 Two recent publications have reported attempts to treat refractory OSSN with intralesional anti-VEGF therapy. One involved a case report of a 79-yearold man with a large sessile papillomatous tumor that did not respond to topical or intralesional IFN-2B, topical MMC, or topical 5-FU, and in whom surgical intervention was not recommended secondary to anticoagulant therapy.47 An intralesional injection of 1.25 mg of bevacizumab was attempted, but after 4 weeks of follow up no change was observed. There were no obvious effects of the treatment noted on pathologic evaluation after excision was finally performed. The second publication, a report on a 2-year trial of subconjunctival ranibizumab as treatment for refractory SCC of the ocular surface included patients who were originally treated with surgery, cryotherapy, topical MMC, and topical IFN-2B; 0.5 mg of ranizumab was injected subconjunctivally every 2 or 4 weeks if the tumor size had remained stable or regressed. Three out of the five patients who were enrolled made it through the 24-month study period with no evidence of recurrence. The average number of injections received in the tumors that responded was just over 20 in the 2 year period. One of the cases had an initial response and then a recurrence, and the last patient demonstrated no tumor regression.48 Neither of the two publications reported any evidence of systemic or severe ocular side effects, but the sample size was small.
Future Directions
As the armamentarium of chemotherapeutic options expands, it is likely other agents will be successfully utilized in the treatment of OSSN. Pegylation and other modifications of interferon may have effects on the efficacy and side-effect profile. A potential, though as of yet untested, therapeutic option for the treatment of OSSN is anti-epidermal growth factor receptor (anti-EGFR) monoclonal antibodies such as cetuximab. Like anti-VEGF antibodies, anti-EGFR agents were originally developed as a solid tumor anti-neoplastic therapy and are currently used in colorectal, ovarian, and non-small cell lung cancer, among others. Also like anti- VEGF therapies they have been shown to be effective against SCCs of the head and neck.49 It is unclear if epidermal growth factor plays a role in the development or growth of OSSN. Several ocular side effects have been reported in the literature resulting from systemic administration of anti-EGFR treatments including dysfunctional tear syndrome, blepharitis, and trichiasis;50 it is unclear if the doses needed for local therapy would result in similar effects. Advances in diagnostic and imaging technologies can also be expected to enhance the quality of care available for ocular surface tumors.
Comments
The increasing evidence described in this paper supporting the use of topical and intralesional medications as adjuvant and primary treatment options appears to be associated with an ongoing shift in the treatment paradigm for OSSN. Compared with the results of an OSSN treatment standard of care survey published in 2005,51 unpublished data from a follow-up 2012 survey shows that there is an overall shift toward greater use of topical agents. In the 2012 survey, 79 % of respondents believed there is sufficient evidence to support the use of topical agent monotherapy, and there was an increase in the acceptance of topical monotherapy in all groups of tumor sizes. While MMC was the topical treatment of choice in the 2005 survey, there seems to be a shift toward utilizing IFN-2B as the initial topical agent, likely as a result of better patient tolerance and decreased side effects.
Although there is a greater acceptance of topical treatment for OSSN, there are no standardized indications secondary to the varying inclusion criteria and clinical presentations in published reports. Sepulveda et al. suggested relative indications could include eyes that have greater than two quadrants of conjunctival involvement, greater than 180 degrees of limbal involvement, extension into the visual axis of the cornea, positive margins after excision, and patients who are unable to undergo surgery.22 Currently it is left to the physician’s discretion when and how to utilize topical therapies, but there appears to be sufficient evidence to support their use in a variety of clinical situations. More research is needed to further elucidate the ideal treatment algorithm for OSSN and to continue the search for better alternative therapies when needed.