Uveal melanoma (UM) is the most common primary intra-ocular malignancy in adults. The incidence of UM ranges from 4.3 to 10.9 cases per million and has remained stable for the past 50 years.1 Presentation is at a median age of 60 years, and men and women are equally affected. UM is a neoplasm that arises from melanocytes in the uveal tract, which comprises the choroid, ciliary body and iris. Choroidal melanomas are the most common and display a discoid, dome-shaped or mushroom-shaped growth pattern. UM have a strong tendency to metastasise to the liver. Over the past decades, ocular ophthalmologists have shifted towards more eye-conserving treatment with the aim of preserving vision. Despite primary treatment, nearly half of the patients eventually die due to metastatic disease and the patient survival has not improved over the past 30 years.2 Metastasising UMs often contain non-random chromosomal aberrations, such as loss of chromosome 3 and gain of chromosome 8q. Research focuses on finding genetic prognostic markers in UM to select those patients at risk of developing metastatic disease. The most recent results are the tumour-specific mutations that have been found in GNAQ, GNA11 and BAP1 genes.3–5 These latest findings can have implications for the future therapy of UM by developing new treatments based on the specific gene content. In this article we will review the diagnosis and current treatment modalities for choroidal melanomas as well as metastases. The future prospects regarding therapeutic options will also be discussed.
Clinical Diagnosis
UM are often noticed at a routine ophthalmic examination since 30 % of the patients have no symptoms.6 If a patient with UM presents with symptoms, these can include blurred vision, floaters, photopsias and visual loss, depending on the size and location of the tumour. In some cases the patient presents with severe ocular pain secondary to inflammation or neovascular glaucoma.
The diagnosis of UM is based on clinical examination with the slit lamp and indirect ophthalmoscope together with ultrasonography (US) of the eye. The majority of UM are pigmented lesions (melanotic), only one-quarter is amelanotic or relatively non-pigmented. The tumours can grow towards the vitreous displaying a discoid, dome-shaped or mushroom-shaped growth pattern. Small melanomas are more difficult to detect than medium- and large-sized melanomas. UM are subdivided according to the apical size of the tumour and the diameter.
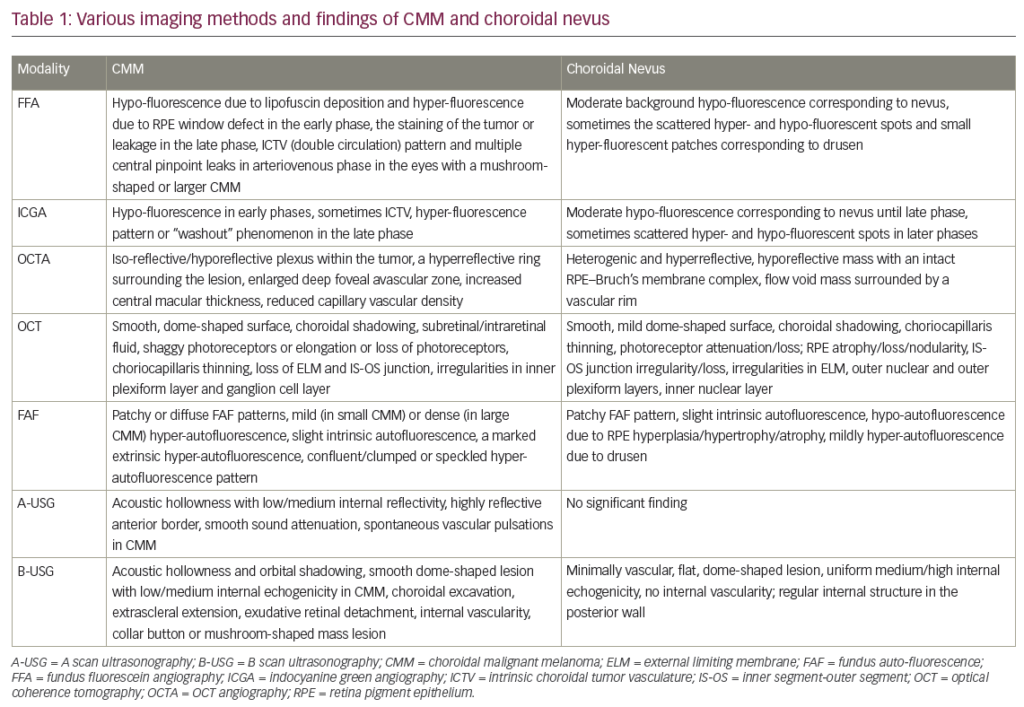
Small melanomas have a diameter of >5 mm with a thickness of 1.0 to 2.5 mm, medium-sized lesions are <16 mm in diameter and 2.5 to 10.0 mm thick and large UM are either >16 mm in diameter with a thickness of >2.0 mm or have a thickness of >10.0 mm regardless of the basal diameter.7 They can appear flat or dome-shaped. It remains challenging for ophthalmologists to differentiate between small choroidal melanomas and choroidal nevi, and the latter are the most important differential diagnosis of UM. Other differential diagnoses for UM are shown in Table 1. In general, choroidal nevi have a <5 mm basal diameter and are minimal in height, <2 mm, although several definitions of nevi have been proposed. To differentiate small UM from other choroidal pathologies, including choroidal nevi, Shields et al. formulated the mnemonic ‘TFSOM’, i.e. ‘to find small ocular melanoma’ to identify indicators of potential malignancy and predict growth.8 The letters indicate Thickness >2 mm, subretinal Fluid, Symptoms, Orange pigment and Margin to the optic disc. If none of the factors are present there is a 4 % chance of growth, while if there are one or two factors present the chance of growth rises to 36 % and >45 %, respectively.9The TFSOM mnemonic was later extended with ‘Using Helpful Hints Daily’ including three features, such as Ultrasound Hollowness (or low acoustic profile), absence of a Halo around the tumour and absence of Drusen.10 Extraocular extension into the orbit can occur at any stage and can be detected with US, computed tomography (CT) and magnetic resonance imaging (MRI). Optical coherence tomography (OCT) and fundus autofluorescence can be useful in differentiating melanomas from other pigmented lesions (e.g. melanocytoma).11Small tumours can be measured and subretinal fluid can be observed with an OCT, whereas orange pigment can be visualised using fundus autofluorescence. Spectral domain OCT can be useful in the detection of subretinal deposits, vitreous seeding and transretinal tumour extension.12 Other ancillary tests include fluorescence angiography (FAG), indocyanine green angiography (ICG), CT and MRI. The diagnostic value of FAG is limited but it can aid in differentiating UM from other lesions. The choroidal vasculature can be visualised with ICG and provides more information than FAG. Often late staining is observed because the indocyanine green leaks in the extracellular space of the tumour.13–15 To detect extrascleral extension, CT and MRI are more sensitive than US.16,17 However, these techniques are not routinely used in the diagnostic evaluation of UM since they are quite expensive.
Table 1: Differential Diagnoses of Uveal Melanoma
Treatment of Primary Uveal Melanoma
The treatment of UM depends on the size and location of the tumour, the secondary effects of the tumour on the eye (for instance, inflammation or neovascular glaucoma), the status of the fellow eye and the patients’ choice. Over the years, eye-preserving therapies have proved to be equally effective in terms of overall patient survival and metastasisfree survival compared with radical treatment.18,19 Therefore, nowadays, radical treatment or enucleation is performed in those cases with larger or advanced melanomas, or when extraocular extension is present.20 A summary of the treatment modalities for UM, together with indications and complications, is listed in Table 2.
The most common treatment among the conservative modalities is brachytherapy. With brachytherapy or local irradiation, radioactive material is placed on the sclera at the location of the tumour. Ruthenium-106 (Ru-106) and iodine-125 (I-125) are frequently used applicators. The plaque-shaped applicator is sutured to the sclera and removed a few days later once the required dose of at least 80 Gy has been delivered to the tumour. Ru-106 applicators have a limited depth of penetration compared with I-125 applicators. Therefore Ru-106 is usually used for tumours with a maximal thickness of 7.0 mm.21,22 Brachytherapy can be combined with transpupillary thermotherapy (TTT) to treat those cases with thicker tumours or to gain more tumour control if there is a suspicion that the tumour margins are not covered with brachytherapy. This is referred to as ‘sandwich therapy’.23,24 TTT is rarely used as a primary treatment; only those patients with small pigmented choroidal melanoma near to the fovea or optic disc may sometimes receive TTT.25 After brachytherapy, radiation-induced complications can occur and include radiation retinopathy, radiation maculopathy, radiation opticopathy, vitreous haemorrhage, cataract and iris neovascularisation leading to neovascular glaucoma.26–28 Local recurrences after brachytherapy have been described in 4–28 %, depending on tumour size and duration of follow up.29–32 Ten to 22 % of the patients eventually have to be secondary enucleated due to radiation-induced side effects.33–36 Heavy particle radiation with proton beam is available in some centres and some administer this treatment to all patients, while others reserve it for patients whose tumour is unsuitable for brachytherapy. Proton beam radiotherapy consists of several steps. First, a clip is sutured to the sclera around the base of the tumour followed by dose admission two weeks later (50–70 Gy relative biological effectiveness in 4–5 fractions).37,38 Proton beam radiotherapy has some advantages compared with brachytherapy and fractioned stereotactic radiotherapy (SRT ) (see below) since the dose reaches the tumour homogeneously and healthy tissue surrounding the tumour can be spared.39 Proton beam radiation enables treatment of choroidal melanomas of all sizes but usually tumours up to 20 mm in diameter and 12 mm thickness are treated. When treating larger or thicker tumours with proton beam, there is a lower chance of preserving vision.40 The local recurrence rate after treatment is 5 % after 10 years and this is in the same range as brachytherapy.30 Secondary enucleation rates are 10–15 % due to local recurrences or complications.41,42 Complications after proton beam radiotherapy include retinal detachment, neovascular glaucoma, cataract, optic neuropathy, maculopathy, vitreous haemorrhage and dryness.37
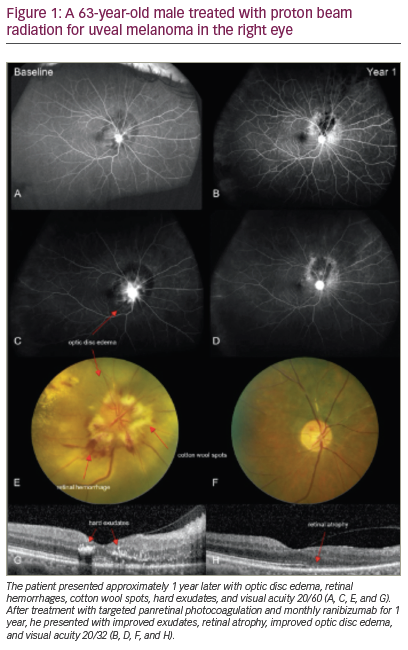
Another eye-preserving option is SRT for the treatment of small and medium-sized posteriorly located melanomas and is becoming available in more centres. The effect of SRT on a medium-sized choroidal melanoma is depicted in Figure 1. One advantage of SRT compared with previously mentioned therapies, is that SRT requires no surgical procedures to determine the tumour localisation and dimensions. The tumour borders are delineated with CT and/or MRI. Stereotactic irradiation can be performed using a gammaknife, a cyberknife or a linear accelerator (LINAC). Most centres administer a total dose of 50 Gy in 4–5 fractions, although some centres prescribe higher doses.43,44 Reported complications are similar to those of brachytherapy and proton beam radiotherapy and can lead to visual impairment and secondary enucleation (3–16 %).43–45 Other radiogenic side effects include conjunctivitis, blepharitis or dry eye syndrome. Studies report similar local tumour control rates – 90 % 5 and 10 years after SRT .4–45
Photodynamic therapy is occasionally used as an alternative treatment for UM. The use of photosensitiser verteporfin has been described in several reports and is being more applied in amelanotic tumours.46-48 Local resection of small iris and ciliary body melanomas are widely carried out. This is not the case for choroidal melanomas. In the past, ophthalmologists were reluctant to operate on choroidal melanomas as they were concerned about manipulation of tumour cells that could lead to an increased risk of metastasis. Currently, due to improved surgical techniques and more insights on tumour progression towards metastatic disease, surgical excision is considered to be a therapeutic option for choroidal tumours. The tumour can be removed in several ways, through the vitreous and retina with a vitreous cutter, endoresection, or through a scleral opening, exoresection (e.g. iridectomy, iridocyclectomy, cyclochoroidectomy, choroidectomy). This way eyes with large melanomas, which otherwise would be enucleated, can be preserved. Surgical resection can serve as primary treatment for UM or additional to another kind of radiotherapy. Often, radiotherapy is administered prior to endoresection and exoresection is followed by treatment with brachytherapy. By using resection as a treatment, radiation-induced problems such as toxic tumour syndrome, a result of radiation-induced necrosis comprising exudative maculopathy, serous retinal detachment, rubeosis and neovascular glaucoma, can be avoided. Another advantage is that, especially with the larger melanomas, tumour tissue is available for prognostification and research.29,49,50
Figure 1: Stereotactic Radiotherapy Effect on Medium-sized Uveal Melanoma
Table 2: Summarised Treatment Options for Uveal Melanoma
Treatment of Liver Metastasis
Despite successful eradication of the ocular tumour, about 50 % of all UM patients develop metastatic disease. Metastasis spread haematogeneously to the liver and death often follows within 1 year if systemic symptoms occur.51 There are no standardised therapies that improve survival in metastatic disease. Systemic treatment options, such as intravenous chemotherapy and immunotherapy, prolong life only rarely.52 Systemic therapy may be more effective if administered early after diagnosis treating micrometastatic rather than macrometastatic disease. Targeted systemic therapies in metastatic UM are currently being investigated. Preclinical studies suggest potential benefit when modulating mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-kinase-AKT (PI3K-AKT) pathways, or receptor tyrosine kinases. The BRAF inhibitor sorafenib was administered to uveal metastatic patients in a phase II trial (www.clinicaltrials.gov). A new adjuvant immunotherapy protocol has been developed, where clinical, histological and cytogenetic factors are used to identify highrisk UM patients and treat these by immunisation with their own trained dendritic cells to prevent future metastatic disease. This multicentre trial is ongoing within our Rotterdam Ocular Melanoma Study group in collaboration with the Department of Tumor Immunology of Radboud University Nijmegen.
There are several locoregional techniques available, for instance, hepatic intra-arterial chemotherapy, chemoembolisation, immunoembolisation and isolated liver perfusion. A partial hepatectomy can be beneficial in some highly selected patients. Surgery in patients with 4 or fewer metastatic lesions, more than 24 months from initial diagnosis to liver metastases and absence of miliary disease (multiple, diffuse, smallsized, dark punctuate lesions on CT) has been associated with a better outcome. A microscopically complete liver resection can increase the survival time. In a retrospective study of 255 patients who underwent surgical resection, the median overall post-operative survival was 14 months.53 In patients who had a microscopically complete liver resection (compared with a microscopically or macroscopically incomplete liver resection) the survival increased to 27 months.
Future Prospects
As mentioned above, studies investigating UM therapies with a targeted approach are ongoing. This is especially interesting since the discovery of mutations in a subset of genes in UM. A vast majority of tumours harbour either a GNAQ or GNA11 (Gα) mutation (up to 83 %).5 These genes are involved in the MAPK pathway and mutations in the Gα genes are considered to be an early event in UM and not related to the tendency to metastasise.4,54,55 One molecule in this pathway is MEK and several preclinical studies have investigated MEK as a potential therapeutic target.56–58
The BRCA1 associated protein 1 (BAP1), is mutated in up to 84 % of the metastasising UM implicating that these mutations play a role in the tumour progression.3 BAP1 plays a role in chromatin, DNA damage response and cell growth mediating deubiquitination of histone H2A and HCFC1.59,60 BAP1 is located on chromosome 3p21,1 and is thought to be a tumour-suppressor gene.60 A recent preclinical study showed that histone deacetylase (HDAC) inhibitors can reverse the effects of BAP1 depletion in UM cells, suggesting that HDAC inhibitors may have therapeutic potential.61
With new technologies, such as Next Generation Sequencing, more is learned about the human genome and new cancer-susceptibility genes in UM, which may serve as targets for new interventions. These molecular approaches will hopefully lead to improved patient survival.