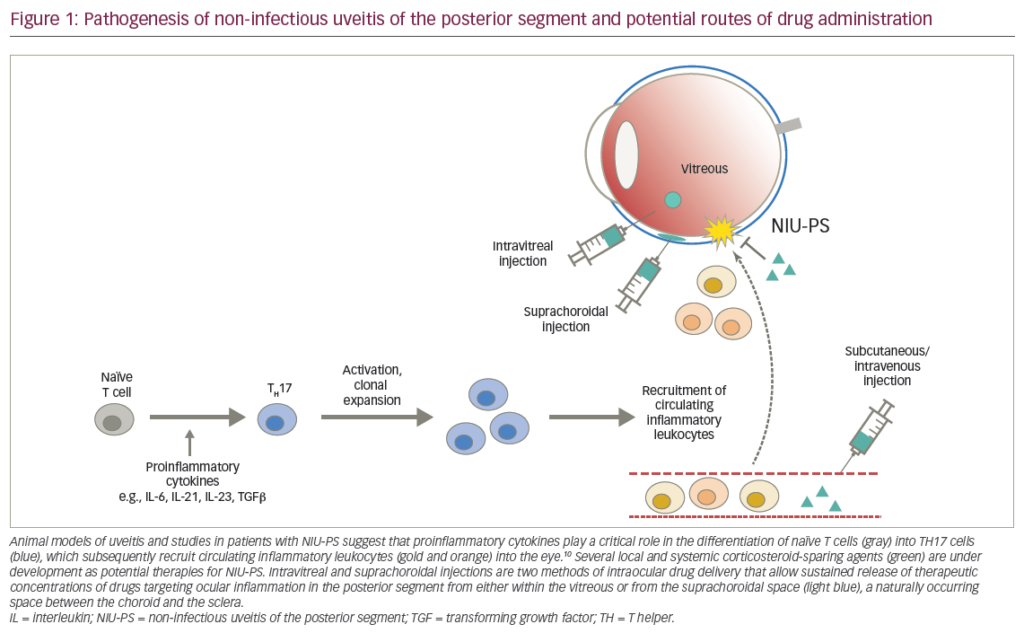
Uveitis is an inflammatory eye disease affecting the iris, ciliary body, and choroid that can lead to symptoms ranging from redness, pain, and blurred vision to markedly diminished acuity in the setting of severe or chronic disease.1 It affects approximately 38,000 Americans per year, with an incidence of 15 cases per 100,000 per year and a prevalence in the US and Europe of 38 per 100,000.2 The broad disease entity of uveitis can be further classified into the anatomical divisions of anterior, intermediate, posterior, and panuveitis.2 Anterior uveitis primarily affects the iris, ciliary body, cornea, or sclera, and usually has a non-infectious—and often idiopathic—etiology.1 Overall, anterior uveitis is the most common form, representing 28–61% of all cases.2 Intermediate uveitis affects the anterior vitreous and pars plana, causing floaters and vision loss from cystoid macular edema.1 Intermediate uveitis is responsible for the smallest proportion of overall cases, ranging from 3 to 17%.2 Most cases are idiopathic (69.1%), but the most common definable etiologies are sarcoidosis (22.2%), multiple sclerosis (8%), and Lyme disease (0.6%).2 Posterior uveitis involves the retina and choroid and accounts for 9.3–38% of all uveitis cases.2 This form of uveitis accounts for more visual loss than other forms, often due to cystoid macular edema, retinal detachment, cataract, glaucoma, subretinal fibrosis, and optic disc atrophy.3 Posterior uveitis is often infectious, with toxoplasmosis and cytomegalovirus representing 24.6 and 11.6% of cases, respectively. Other etiologies are idiopathic, systemic lupus erythematosus (SLE), birdshot retinochoroidopathy, sarcoidosis, Adamantiades-Behcet’s disease (ABD), and syphilis.2 Finally, panuveitis affects all three sections of the uveal tract and is responsible for 7–38% of all cases.2 The most common cause is idiopathic (22.2%), followed by sarcoidosis (14.1%) and multifocal choroiditis (12.1%).
Current Medical Therapeutic Options
Corticosteroids
The mainstay of non-infectious uveitis treatment is corticosteroids, which are administered in three forms: topically, locally via sub-Tenon’s or intravitreal injection, and systemically.4 Each of these treatment modalities has strengths and weaknesses that must be weighed in order to individualize treatment for a particular patient. Topical steroid drops and ointment are primarily useful for anterior uveitis or as adjunct therapy along with systemic treatment for panuveitis, as penetration into the posterior segment is minimal.4 Local injection of long-acting steroids such as triamcinolone results in therapeutic concentrations in the posterior segment, and therefore can be useful for treatment of posterior uveitis in some individuals.3 The relatively low systemic concentration of steroids achieved by this method spares patients from the usual complications of systemic steroid therapy.3 However, there are disadvantages to this form of therapy, including the necessity of repeat local injections as the steroid concentration in the posterior segment declines and inflammation recurs after approximately three months.4 These patients may suffer worse outcomes than patients treated with systemic steroids due to visual loss in the interval between steroid injections.3 In addition, intraocular steroid injections can lead to increased intraocular pressure, ptosis, and strabismus, and carry a risk of globe penetration.3 Many patients with posterior uveitis or panuveitis depend on systemic steroids to achieve longterm control of intraocular inflammation.4 Oral prednisone is most often used, starting at a dosage of 1–2mg/kg/day until inflammation abates, and then tapered gradually to a maintenance level of 10mg/day or less.3 In cases of vision-threatening inflammation, pulsed intravenous methylprednisolone 1–3g for three days can be used for rapid resolution; patients may be switched to lower doses of oral prednisone thereafter.3 Due to the recurrent nature of uveitis once steroids are discontinued, many patients must be maintained on significant doses (>10mg/day) of long-term oral prednisone in order to suppress active inflammation. Unfortunately, this course of treatment can lead to numerous adverse effects, including Cushingoid features, hyperglycemia, osteopenia and osteoporosis, and bone marrow suppression.
Immunomodulatory Therapy—Steroid-sparing Agents
Immunomodulating drugs may be added to the corticosteroid regimen to eliminate the need for high doses of systemic steroids, or used alone as steroid-sparing agents when steroids are not tolerated. Pharmacological agents used as part of immunomodulatory therapy (IMT) can also have potential adverse events, and thus must be used by physicians who have had extensive experience in administering them.
Antimetabolites
Methotrexate is a folic acid analog that inhibits dihydrofolate reductase, which is necessary for DNA and RNA synthesis. Its role in posterior uveitis, panuveitis, or selected cases of severe anterior uveitis is primarily as a steroidsparing agent, and it is often used after the disease has been controlled by a high dose of systemic steroids.3 Methotrexate (Rheumatrex®) is given weekly, and adverse effects include gastrointestinal upset, reversible hepatotoxicity, pulmonary fibrosis, and teratogenicity.3,4 Azathioprine (Imuran®) is a purine analog that has been used for the treatment of Behcet’s syndrome, Vogt- Koyangi-Harada (VKH) syndrome, and sympathetic ophthalmia.3 Mycophenolate mofetil (MMF; Cellcept®) is also a purine synthesis inhibitor, originally developed for the prevention of kidney transplant rejection. It has been used for the treatment of posterior uveitis, panuveitis, and severe anterior uveitis requiring systemic therapy. MMF has its own treatmentlimiting side effects, including diarrhea;4 however, among the three antimetabolites commonly used to manage uveitic and ocular inflammatory diseases (methotrexate, azathioprine, and MMF), MMF is often more effective in achieving disease quiescence and is well-tolerated by patients.
T-cell Inhibitors/Calcineurin Inhibitors Cyclosporine (Neoral®), an inhibitor of the inflammatory cytokine interleukin- 2, has been shown to be effective in the treatment of ABD, although it is often combined with corticosteroids for optimal results.3 Its usefulness is limited by nephrotoxicity and hypertension. Other agents in this class include tacrolimus (Prograf®). LX 211, an agent currently being evaluated in clinical trials as a potential therapeutic option for uveitic diseases, is also a calcineurin inhibitor.
Alkylating Agents
Alkylating agents are known for their potency in achieving disease quiescence and in putting the disease into remission. However, they must be used only when truly indicated and, given their significant side effects, with great caution. Cyclophosphamide (Cytoxan®), an alkylating agent used in the treatment of neoplastic disease, can be used for Wegener’s granulomatosis and necrotizing scleritis, though it is known to cause sterility and hemorrhagic cystitis, which may be associated with bladder cancer.3 Chlorambucil (Leukeran®), another alkylating antineoplastic agent, has also been used in the treatment of posterior uveitis, including ABD.3
Biologics
A newer approach to the treatment of uveitis is cytokine inhibition. Cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-2 have been implicated in the pathogenesis of inflammatory disease. Selective inhibition of specific cytokines is expected to provide focused inhibition of the immune system. The various cytokine inhibitors exhibit multiple mechanisms of action. Cytokines may be bound by a soluble receptor, making them transiently unavailable. Antibodies against selected cytokines can permanently remove the cytokine from the circulation. Alternatively, antibodies against the cytokine receptor can prevent circulating cytokines from binding to the receptor, inhibiting the inflammatory response. Several cytokine inhibitors—infliximab (Remicade®), daclizumab (Zenapax®), and adalimumab (Humira®)—are employed in the treatment of chronic noninfectious posterior uveitis; they are often used in cases that have been resistant to other treatments. Their efficacy and ability to provide long-term control of inflammation in uveitis have been demonstrated in a number of small studies, although results from controlled clinical trials are necessary to determine their roles in the management of uveitic diseases.
Sustained-release Devices
The desire to achieve long-term control of the disease in order to avoid the cumulative damage resulting from recurrent attacks of inflammation, as well as the wish to avoid adverse events secondary to systemic therapy with corticosteroids or IMT, have led to the development and employment of sustained-release devices.
Fluocinolone Acetonide Intravitreal Implant
During the past two decades, significant progress has been made toward delivering pharmacological agents using a constant, long-term approach. The availability of the ganciclovir device (Vitrasert®) has saved the vision of many patients who suffer from cytomegalovirus retinitis. The 0.59mg fluocinolone acetonide device Retisert®, available since 2005, has become the first drug or device approved by the US Food and Drug Administration (FDA) for the treatment of non-infectious uveitis. The constant delivery of medication in a sustained device such as the Retisert has been shown to decrease the recurrence of inflammation, the need for adjunctive therapy, and the necessity of systemic therapy compared with eyes that have not received the implant. Thus, sustained-release devices, with proper therapeutic agents, are likely to help physicians and patients achieve the goal of no tolerance for any degree of inflammation and to decrease or prevent risk of cumulative damage. Sustained-release devices may also improve patient compliance relative to systemic therapy, as patients may be less compliant with systemic drugs when they have to experience the adverse side effects. Nevertheless, although compliance is less of a concern with a sustained-release device than with systemic medications, it remains a significant factor in managing patients with uveitis. Patients still need to be monitored closely for intraocular pressure. Therefore, a patient who is not compliant with follow-up visits is not a good candidate for a steroid-release device.5 In such cases, one could be simply exchanging one type of blinding condition (uveitis) for another (untreated glaucoma); the ophthalmologist may be able to maintain the inflammation in quiescence, but the patient may suffer from glaucomatous optic neuropathy.5 Thus, it is critical that the ophthalmologist has a direct, honest, and comprehensive discussion with the patient regarding the potential risks and benefits of each therapeutic option.
Summary
The management of uveitis is challenging because of its protean etiologies and its manifestations, both ocular and systemic. If the etiology of the uveitis is infectious, proper therapy with antibacterial, antifungal, or antiparasitic agents often leads to resolution of the uveitis. When the etiology is immunologically driven, chronic inflammation can lead to cumulative damage that eventually causes significant visual loss. It would be beneficial to patients with chronic uveitis for ophthalmologists to recognize recurrent inflammation before cumulative damage occurs so that appropriate therapy, systemic medications, or use of sustained-release devices can be initiated in a timely manner. Being aware of the detrimental effects of cumulative damage and the principle of ‘zero tolerance to any degree of inflammation’ will enable the ophthalmologist to help preserve the vision of many patients with uveitis. In choosing a therapy, the physician needs to be vigilant about the risk of cumulative damage that may result from recurrent inflammation after a treatment approach. The physician also needs to recognize that cumulative damage could occur in patients treated with oral corticosteroids, often because the tolerable dose of corticosteroids is too low to attain adequate inflammatory control. IMT or biologics can then be considered to achieve disease quiescence; however, patients must be monitored carefully for adverse events. The 0.59mg fluocinolone acetonide device is estimated to last for approximately 30 months, but the patient may not have achieved disease quiescence at 30 months. In all instances, physicians should constantly bear in mind the goal of avoiding cumulative damage and should provide additional treatments, such as implanting a second sustained-release device, in order to achieve this goal.