Age-related macular degeneration (AMD) is a chronic deterioration and dysfunction of the outer retinal tissue and Bruch’s membrane (BrM). It is the leading cause of vision loss in people older than 60 years and is estimated to affect 288 million people by 2040 with 39 million new cases by 2050.1,2 AMD has the highest economic burden among vision-impairing diseases.3 There are two distinct types of AMD, described as either non-exudative (‘dry’ AMD) or exudative/neovascular (‘wet’ AMD/nAMD). Notably, these two types are not mutually exclusive, as dry AMD, at any stage, can eventually develop into wet AMD.4
Dry AMD is clinically recognized first by the presence of drusen, which are collections of extracellular debris consisting of lipids and proteins beneath the retinal pigmented epithelium (RPE) and in BrM.5 While subretinal deposits can be a benign finding in normal ageing (i.e. laminar deposits), pathological studies using electron microscopy are more specific for the location of these deposits and the correlation with definitive diseases (i.e. linear deposits and drusen).6,7 Specific clinical classifications, including age, size of the deposits and pigment abnormalities, are used to determine the severity of drusen and their risk of progression to nAMD.8,9 Dry AMD is further divided into three stages: early, intermediate and advanced, each involving increasing alterations to the outer retina and subsequent vision loss. During the earliest stages of AMD, symptoms may not be present. Signs of central vision loss progress slowly, manifesting as mild blurriness and progressing to metamorphopsia as AMD worsens into later stages. Risk factors for AMD include age older than 60 years, smoking, previous cataract surgery, family history of AMD, increased body mass index (BMI), hypertension, cardiovascular disease and genetic predisposition involving complement system-related genes.10 As no U.S. Food and Drug Administration (FDA)-approved treatments for early and intermediate stages of AMD exist, modifiable risk factors, such as smoking, hypertension, and increased BMI, must be identified and addressed.11 In high-risk individuals with advanced AMD, the benefits of vitamin supplementation proposed by the Age-Related Eye Disease Study (AREDS; ClinicalTrials.gov identifier: NCT00000145) have significantly reduced the odds of progression to nAMD.12 nAMD is managed by the long-term administration of intravitreal injections of anti-vascular endothelial growth factor (anti-VEGF) agents under the treat-and-extend protocol.13,14
The most severe form of late dry AMD is geographic atrophy (GA). GA occurs when photoreceptor cells in the outer retina in the macula gradually deteriorate and die, leading to well-defined, enlarging atrophic lesions and depigmented regions. It has been seen that these lesions grow at the same rate in both eyes in bilateral GA.15 Most recent data compilation states that nearly 20 million individuals in the USA have AMD.16 Recent literature also suggests that approximately 20–30% of eyes with AMD will develop GA in the advanced stage.17 GA can also develop in wet AMD as seen in a cohort analysis of participants with nAMD in the CATT study (Comparison of Age-related macular degeneration Treatments Trials; ClinicalTrials.gov identifier: NCT00593450), which showed that GA occurred in approximately 18% of their participants after 2 years.18 In a UK study, the median time for progression to legal blindness in patients with GA was reported to be 6.2 years.19 Demographic investigations into GA reveal a noteworthy association with advanced age, which often accompanies a myriad of concurrent health challenges in afflicted individuals.20,21 These challenges manifest in various aspects of daily life, including the ability to operate a vehicle, engage in routine activities and manage the financial burdens of increased healthcare costs. The specific underlying disease process of GA has yet to be fully uncovered. However, the aberrant complement system activation has been indicated as a cause in past investigations.22,23
Findings of GA are typically characterized and documented by lesion size, location, growth rate, best corrected visual acuity (BCVA) loss and low-luminance visual acuity (LLVA) loss.24 GA typically appears in the extrafoveal region, later progressing to the foveal centre with a median timespan of 1.4–2.5 years.25 In cases of subfoveal GA (affecting the foveal centre), vision loss is typically more pronounced and directly affects central vision. Patients may experience blurred or distorted central vision. Nonsubfoveal GA, while not directly affecting central vision, may lead to disturbances in peripheral or paracentral vision. Patients with nonsubfoveal GA have preserved visual acuity but may experience difficulties in up-close activities due to paracentral scotomas. Fleckenstein et al. state in a review article that the median GA lesion growth rate is 1.78 mm2/year.24 Sunness et al. reported the median GA lesion growth rate to be 2.1 mm2/year, with similar rates seen in both eyes.26 Both the National Eye Institute and the FDA have accepted the decrease in atrophic lesion growth as a primary endpoint for clinical trials.4,27 Although the diagnosis of GA is clinical, a multimodal approach with fundus autofluorescence (FAF) and optical coherence tomography (OCT) is highly useful to help diagnose and monitor progression.28 FAF can reveal hypoautofluorescent RPE tissue as dark areas, indicating the absence of healthy lipofuscin-producing cells. FAF can also show hyperautofluorescence in the junction between atrophic and healthy tissue, indicating the expulsion of lipofuscin from RPE cells during the process of dying.29,30 The absence of hyperautofluorescence at the borders of atrophic areas generally indicates that the atrophy is due to retinal diseases other than AMD. Recent consensus suggests using OCT to record baseline and to track disease progression with evidence that OCT is more sensitive to detecting GA in patients being treated for nAMD.31–34 OCT produces high-definition images, which can be used to identify and characterize morphological changes in GA, such as tissue loss in RPE and outer retinal layers, photoreceptor loss, intraretinal fluid, hypertransmission of BrM and choroidal capillaries, and splitting of the RPE and BrM. Apart from imaging, visual function tests are an important tool to assess real-world consequences on patients. Vision charts may not be adequate, as diseased areas and scotomas are located outside the fovea and the retinal dysfunction can be missed to identify. Instead, specialized reading charts that can measure reading speed are more favourable in testing for deficits. Macula stimulation in multiple areas using a microperimeter and patient feedback can better evaluate vision as well as test for LLVA and contrast sensitivity.35
Prior to 2023, management options for GA were primarily supportive, as there were no specific treatments available for this advanced stage of dry AMD. Regular monitoring is a key part of managing GA, with patients undergoing frequent check-ups to assess disease progression. In February 2023, the FDA approved pegcetacoplan (SYFOVRE™, Apellis Pharmaceuticals, Waltham, MA, USA) as the first-ever medication for the treatment of GA associated with dry AMD. The DERBY (ClinicalTrials.gov identifier: NCT03525613) and OAKS (ClinicalTrials.gov identifier: NCT03525600) trials are phase III studies that assessed the efficacy of pegcetacoplan and have substantiated its ability to decelerate the advancement of dry AMD, with the recommended dose being 15 mg intravitreal injections every 25–60 days.36 More recently in August 2023, avacincaptad pegol (IZERVAY™, Astellas Pharma, Inc., Tokyo, Japan) was also approved for the treatment of GA after supportive findings from the GATHER1 trial (ClinicalTrials.gov identifier: NCT02686658) and the GATHER2 trial (ClinicalTrials.gov identifier: NCT04435366). The current recommended dose for avacincaptad pegol is 2 mg via intravitreal injection to each affected eye approximately every 28 ± 7 days for up to 12 months.37
Methods
We conducted a database search from 2005 to 2024 for publications related to AMD and GA by selecting open-access publications primarily from the PubMed/PMC/MEDLINE databases, the IOVS.org database, the WithPower.com database and ClinicalTrials.gov. Key terms searched included a combination of ‘macular degeneration’, ‘treatment’, ‘management’, ‘geographic atrophy’, ‘pegcetacoplan’, ‘avacincaptad pegol’, ‘complement’, ‘study’, and ‘trial’. Various presentations from physicians, researchers, pharmaceutical companies and conferences (hosted by the American Academy of Ophthalmology, American Society of Retina Specialists, The Retina Society and The Macula Society) were also included in the literature search. This review compiles and provides a summary of current and recent complement system-based therapeutic approaches for GA.
Acknowledgement of limitations
It is essential to acknowledge the inherent limitations stemming from the sources we have referenced as some are cited works drawn from retinal conference abstracts and presentations by pharmaceutical companies. These sources might present preliminary or truncated findings and usually have a less rigorous peer-review process than that which typically characterizes full research publications, potentially impacting the depth and reliability of the data presented. Furthermore, as the topic of GA management continues to evolve, it is possible that future long-term studies could yield findings that differ from the current observations. Our review aims to provide a comprehensive overview of the existing knowledge while recognizing the potential for forthcoming research to provide more nuanced insights into the effectiveness and safety of the discussed therapies.
Role of the complement system in geographic atrophy
The complement system works to eliminate pathogens and damaged cells within an organism by supporting the functions of antibodies and phagocytic cells. The eyes are immune privileged, with the dominant immune defence being only the complement system and innate immune systems. A combination of complement system, perivascular macrophages and microglia plays a crucial role in retinal vascular homeostasis, retinal tissue integrity and clearance of waste.38–40 In the context of AMD, a substantial proportion of genetic risk variants are concentrated within genes associated with the alternative pathway of the complement system.41 Notable elevations in complement activation products, specifically C3d/C3 ratio, are seen in individuals afflicted by AMD.23 A clear consensus has not been reached regarding which complement pathway dysregulation is predominantly responsible for AMD and GA. Evidence also indicates that the accumulation of C1q aligns with its established role in synapse elimination and the neurodegenerative processes seen in other neurodegenerative disorders.42 Consequently, targeting complement factors has surfaced as a promising strategy for addressing GA. The currently approved complement inhibitors show strong efficacy in decreasing the growth rate of atrophic lesions in severe AMD. Challenges with this group of medications, as seen in clinical trials, include conversion into choroidal neovascularization (CNV) in eyes without CNV at baseline, less efficacy in more severe and later disease stages and pharmacokinetic limitations with intravitreal injections.43
Pegcetacoplan injection
Pegcetacoplan injection secured FDA approval on 17 February 2023,44 which was supported by positive findings from the phase III, double-masked DERBY (n=621) and OAKS (n=637) trials, with a primary endpoint being changes in the total area of GA lesions (calculated from the slope value) at 24 months from baseline as measured with autofluorescence.45 Pegcetacoplan works by inhibiting complement factor C3 (Figure 1). Both trials consisted of eyes with subfoveal and nonsubfoveal lesions. At 24 months, the DERBY trial demonstrated that both monthly and once every other month (EOM) treatment arms showed a slower lesion growth of 19% (p=0.0004) and 16% (p=0.0030), respectively, compared with the sham group. Similarly, the OAKS trial also showed that the treatment decreases the growth rate of lesions compared with the sham: reduction of 22% (p<0.0001) in those treated monthly and 18% (p=0.0002) in those treated EOM. Notably, growth rates of GA lesions reduced the most in both treatment arms between months 18 and 24 in both DERBY and OAKS trials. Findings at 36 months are reported in the GALE extension trial (ClinicalTrials.gov identifier: NCT04770545), where at this timepoint, lesion size continues to show divergence with a growth rate reduction of 35 and 24% for monthly and EOM administered pegcetacoplan, compared with the projected growth rate of the control group. Additionally, nonsubfoveal lesions in treated eyes experienced greater reductions than those seen for subfoveal lesions.46 Furthermore, pegcetacoplan exhibited favourable impacts on visual function and quality of life of patients, especially in those with extrafoveal lesions. The treatment also demonstrated a significant reduction in the loss of photoreceptor and retinal RPE cells. As with many intraocular therapies, pegcetacoplan carries the potential for mild-to-severe side effects. At 18 months, nAMD developed in 12% (p<0.01 versus monthly; p<0.0001 versus sham), 7% (p<0.0214 versus sham) and 3% in the monthly, EOM and sham groups, respectively, indicating a statistically significant increased likelihood for conversion into CNV with the treatment (these specific p-values were not officially reported by Apellis but were calculated by the authors using Z-statistics, comparing two population proportions).45 In recent reports, a total of 10 real-world cases involving pegcetacoplan injection treatment-developed retinal vasculitis have been verified as of October 2023. These patients experienced visual symptoms with a median onset occurring approximately 1–2 weeks after treatment. To date, over 24,000 injections have been administered, with the incidence of retinal vasculitis remaining exceptionally rare, estimated at 0.01% per injection.
Figure 1: The mechanism of action of various complement inhibitors on the complement activation cascade
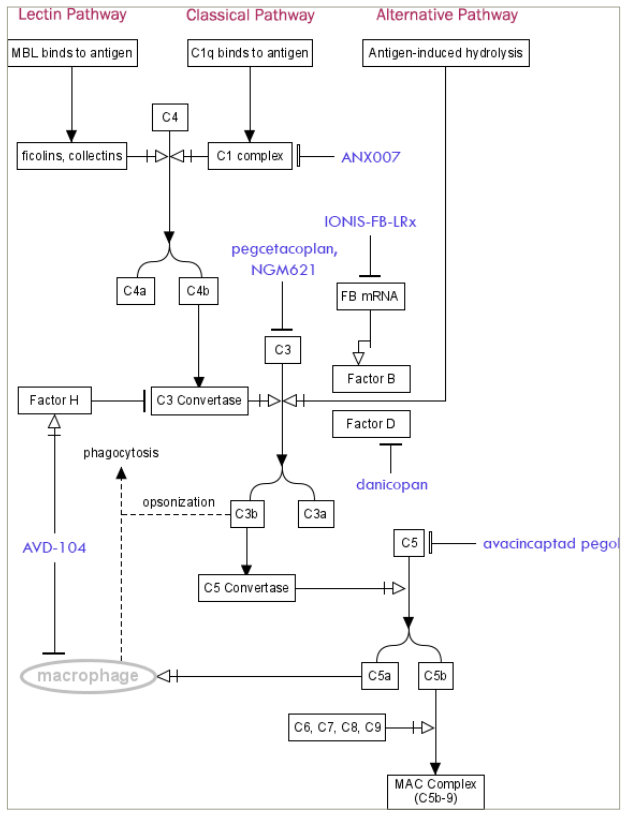
Figure was generated by the author using PathVisio.
C = complement factor; FB = factor B; MAC = membrane attack complex; MBL = mannose-binding lectin; mRNA = messenger ribonucleic acid.
Avacincaptad pegol
Avacincaptad pegol is an intravitreally delivered inhibitor that targets complement factor C5 (Figure 1). The approval of the drug by the FDA on 4 August 2023 was supported by findings from the GATHER1 and GATHER2 phase III clinical trials.47 The phase II/III GATHER1 trial assessed the safety and efficacy of avacincaptad pegol at 12 months in patients with non-foveal GA lesions in part within 1.5 mm from the foveal centre point.34 The results revealed that monthly doses of 2 and 4 mg of avacincaptad pegol led to a reduction in GA growth rates by 27.4% (p=0.0072) and 27.8% (p=0.0051), respectively. Notably, both doses exhibited a noticeable divergence in growth curves from the sham group as early as month 6. This separation persisted and demonstrated a progressive increase in therapeutic efficacy for both dosages over the course of 18 months.48 Expanding upon these findings, the phase III GATHER2 trial aimed to assess the effects of avacincaptad pegol for 24 months.49 The patients of the GATHER2 trial were randomly assigned to receive either avacincaptad pegol 2 mg (n=225) or a sham treatment (n=223). The primary endpoin t measured GA lesion size using FAF. From baseline to month 12, avacincaptad pegol 2 mg exhibited a mean lesion growth rate of 1.745 mm2/year, while the sham group recorded 2.121 mm2/year. This translated to a growth rate difference of 0.376 mm2/year (p=0.0027), indicating a statistically significant reduction of 18% in GA lesion progression compared with the sham group. Favourable results continue at 24 months after re-randomizing the treatment group into monthly and EOM injections.50 Monthly and EOM injections displayed a reduction in growth rate of 14 and 19%, respectively, compared with the sham group. The safety profile at 24 months continues to exhibit minimal concerns. CNV conversion at 24 months for the treatment pool was similar to sham, with the incidence of CNV in the EOM group being greater than sham.
Investigation of complement system inhibitors in clinical trials
The discussion will focus on results from several recent and ongoing investigational complement inhibitor therapies (Table 1), including, in order of their commencement, GOLDEN (ClinicalTrials.gov identifier: NCT03815825), CATALINA (ClinicalTrials.gov identifier: NCT04465955), ARCHER (ClinicalTrials.gov identifier: NCT04656561), GALE extension trial (ClinicalTrials.gov identifier: NCT04770545), Danicopan (ClinicalTrials.gov identifier: NCT05019521) and SIGLEC (ClinicalTrials.gov identifier: NCT05839041).44,47,51–57
Table 1: The current U.S. Food and Drug Administration-approved and investigational complement system inhibitors for geographic atrophy secondary to age-related macular degeneration
|
Drug |
Trial Number |
Sponsor |
Route/mechanism |
Approval status |
|
Pegcetacoplan |
DERBY (NCT03525613) OAKS (NCT03525600) GALE extension trial (NCT04770545) GARLAND (NCT06161584) |
Apellis Pharmaceuticals, Inc. |
Intravitreal C3 inhibitor |
Approved by the FDA as of 17 February 2023.44 Phase IV trial began September 2023 and is ongoing51 |
|
Avacincaptad pegol |
GATHER1 (NCT02686658) GATHER2 (NCT04435366) NCT05536297 |
IVERIC Bio, Inc. |
Intravitreal C5 inhibitor |
Approved by the FDA as of 4 August 2023.47 Phase III extension trial began September 2022 and is ongoing52 |
|
ANX007 |
ARCHER (NCT04656561)
|
Alexion Pharmaceuticals, Inc. |
Intravitreal C1q complex inhibitor |
Phase III ARROW trial to initiate in mid-202453 |
|
Danicopan/ALXN2040 |
NCT05019521 |
Alexion Pharmaceuticals, Inc. |
Oral factor D inhibitor |
Phase II in progress54 |
|
IONIS-FB-LRx |
GOLDEN (NCT03815825) |
lonis Pharmaceuticals, Inc. |
Subcutaneous ligand-conjugated antisense oligonucleotide against factor B mRNA |
Phase II in progress55 |
|
AVD-104 |
SIGLEC (NCT05839041) |
Aviceda Therapeutics, Inc. |
Intravitreal factor H stimulus, macrophage modulator |
Phase II/III; part 1 completed and part 2 in progress56 |
|
NGM621 |
CATALINA (NCT04465955) |
NGM Biopharmaceuticals, Inc. |
Intravitreal C3 inhibitor |
Phase II results released on 17 October 2022.57 Drug development has ceased |
C = complement factor; FDA = US Food and Drug Administration; mRNA = messenger RNA.
IONIS-FB-LRx
Developed by Ionis Pharmaceuticals, Inc., IONIS-FB-LRx is an antisense oligonucleotide that inhibits the production of plasma factor B (FB), which was originally investigated in a phase II clinical study aimed at assessing the efficacy and safety in adults with primary immunoglobulin A nephropathy. The results demonstrated that IONIS-FB-LRx, administered subcutaneously, effectively reduced plasma FB levels in a dose-dependent manner, achieving reductions of approximately 56 and 72% following 36 days of multiple subcutaneous administrations of 10 and 20 mg, respectively.58 No clinically significant safety concerns were observed. Consequently, the GOLDEN study, a double-masked, placebo-controlled phase II trial, is aimed to evaluate the efficacy of IONIS-FB-LRx in reducing GA lesions over a 12-month period.59
NGM621
The CATALINA trial, conducted by NGM Biopharmaceuticals, Inc., was a phase II clinical investigation aimed at assessing the efficacy and safety of NGM621, an intravitreally delivered humanized immunoglobulin G1 monoclonal antibody that inhibits C3 (Figure 1).60 The administration of NGM621 every 4 (q4) weeks (n=108) and every 8 (q8) weeks (n=104) via intravitreal injection revealed a GA lesion area growth rate of 6.3% (p=0.435) and 6.5% (p=0.422), respectively, compared with that of the sham group (n=106) at 52 weeks.57 The reduction of GA lesion area from baseline for q4 and q8 treatment arms was 7.4% (p=0.341) and 6.6% (p=0.400), respectively, compared with that of the sham group. While the statistical significance was not achieved in either treatment arm, a post hoc analysis of patients with baseline GA lesion areas of 4.17–9.64 mm2 (as opposed to the original inclusion criteria of 2.5–17.5 mm2 at baseline) showed that the q4 treatment arm resulted in a 21.9% (p=0.020) reduction in growth rate and a 20.6% (p=0.024) reduction in lesion area from baseline.61 Post hoc analysis results may suggest that NGM621 may be more clinically beneficial in patients with less severe disease at baseline. NGM621 also exhibited a favourable safety profile, with fewer instances of CNV conversions compared with those in the sham group.
ANX007
The ARCHER trial is a phase II study assessing the effectiveness and safety of ANX007 (Annexon, Inc., Brisbane, CA, USA), an intravitreally administered monoclonal antibody that inhibits C1q3 (Figure 1).62 The study contains a sham group and two treatment groups: one treated monthly and another treated EOM. The primary endpoint was the change in GA lesion size at 52 weeks. Secondary functional endpoints include BCVA, LLVA and low-luminance visual deficit (LLVD). The findings show that all groups displayed an increase in lesion growth and also that ANX007 did not elicit a statistically significant difference in change in lesion size when compared with the sham group: 6.2% (p=0.526) less than sham within the monthly treatment arm and 1.3% (p=0.896) less than sham within the EOM treatment arm. Secondary endpoints showed more favourable results. A BCVA loss of ≥15 letters was observed in 5 out of 89 (p=0.0021) patients within the monthly treatment group, which was significantly less than the 19 out of 89 patients within the sham group. Although the outcomes pertaining to LLVA did not demonstrate statistically significant protective shifts, LLVD loss of ≥15 letters occurred in 5 out of 53 (p=0.0305) patients within the monthly treatment group, as opposed to 13 out of 51 patients within the sham group. At 52 weeks, monthly administration of ANX007 showed a 72% (p=0.0060) risk reduction of ≥15 BCVA letter loss in the fellow eye of patients with bilateral GA. While the primary endpoint of reducing GA lesion size was not achieved, the clinical benefit of ANX007 in reducing BCVA loss and protecting low-light visual function remained consistent across various baseline patient characteristics, such as lesion size, lesion location and multifocality. Phase III of the study will begin mid-2024. A head-to-head trial with pegcetacoplan is planned for late-2024.
Danicopan
Danicopan (ALXN2040, Alexion Pharmaceuticals, Boston, MA, USA) is an oral complement factor D inhibitor. Preclinical studies have aimed to assess drug penetration and sustainment in retinal tissue. Melanin has been shown to act as an effective medium for drug binding and ocular delivery owing to its high concentrations in the uvea and posterior pole, serving functions related to light absorption, antioxidation and metabolic support for the RPE.63–65 Previous investigations with danicopan leveraged melanin as a vehicle to achieve proper penetration through the blood–retina barrier and sustained compartmentalization and release of the drug.66,67 Danicopan is currently in phase II studies.
AVD-104
SIGLEC is a phase II trial evaluating the safety and efficacy of AVD-104 (Aviceda Therapeutics, Inc., Cambridge, MA, USA), an intravitreally injected nanoparticle that inhibits macrophage function and complement cascade activation.68,69 Preclinical studies of AVD-104 offered insight into its anti-inflammatory and protective effects.70 Rat eyes treated with AVD-104 displayed a more preserved outer nuclear layer (p<0.01) of the retina and reduced tumour necrosis factor-α (p<0.0001) after bright-light damage compared with the sham group. Rats treated with AVD-104 and then afflicted with laser-induced CNV showed a smaller CNV lesion size and less C5b–9 (membrane attack complex) than those given sham. Phase II of this study commenced in mid-2023 with two ongoing parts.71 Part 1 followed patients treated with escalating doses of AVD-104 for 3 months to evaluate the drug safety in which all 30 participants displayed no severe adverse reaction.72 Part 2 will compare the growth rate of GA lesions over 12 months in 300 patients treated with two different monthly dosages of AVD-104 with that of those given an active comparator.
Discussion
GA is a visually debilitating condition with significant psychosocial, economic and health burdens. No current treatment can permanently halt the disease progression, so medical options that slow the degenerative processes become crucial in extending the vision potential and structural anatomy of the affected eyes. The well-established role of the complement system in mediating inflammation and contributing to retinal degeneration has created an opportune environment for drug development, offering a wide array of complement factors as potential therapeutic targets. Prior to the approval of pegcetacoplan and avacincaptad pegol, physicians and patients faced a daunting challenge in determining suitable clinical management strategies and exploring alternative options to alleviate the burden of vision loss due to this condition.
In randomized clinical trials, both pegcetacoplan and avacincaptad pegol show evidence of slowing the progression of lesions associated with the disease. These FDA-approved drugs have not shown evidence for improving visual acuity but demonstrate an anatomic benefit, which is believed to be correlated with the preservation of longer-term visual function and acuity. Both drugs have demonstrated efficacy and safety, whereas prior attempts at developing complement inhibition for GA in the Chroma/Spectri trials (ClinicalTrials.gov identifier: NCT02247479; ClinicalTrials.gov identifier: NCT02247531) have been unsuccessful.73 The ophthalmic community expects that more drugs with similar mechanisms will prove to be efficacious.
As evident in the CATALINA trial, one of the challenges associated with the effectiveness of complement inhibitors includes limited therapeutic outcomes in eyes with more severe diseases. This highlights the importance of a thorough examination and imaging study for early diagnosis and adequate follow-up. The findings from the ARCHER trial highlight that although treatment may not consistently achieve significant reductions in lesion growth rate, this should not exclude the potential for improvements in visual function. While endpoints in current trials are primarily focused on changes in lesion size, recent imaging-guided studies highlight the importance of evaluating changes in ellipsoid zone integrity, as this may also be a biomarker in GA progression and functional vision loss.74–76
Similarly, the ReCLAIM-2 (ClinicalTrials.gov identifier: NCT03891875) trial, investigating other drugs, such as mitochondrial modulator elamipretide, did not meet its primary endpoints in reducing atrophic lesion growth but had demonstrated statistically significant LLVA letter/line gains and decreased ellipsoid zone attenuation.77,78 Despite the abundance of potential targets within the complement system, the specific interactions within each pathway and the complement factors exerting the greatest influence on disease progression remain yet to be adequately elucidated. Likewise, potential chimeric complement inhibitors having multi-targeting functions against inflammatory or degenerative pathways may result in more pronounced therapeutic effects.
Gene variants of complement factors influence the disease progression in patients and their response to treatment as suggested in the MAHALO trial (ClinicalTrials.gov identifier: NCT01229215), where lampalizumab had an exaggerated therapeutic effect on patients with polymorphisms in complement factor I compared with the entire cohort.79 Genetic studies uncovering the variants in complement system factors may provide information on which patients will benefit the most from treatment. Variability in drug dosing and dosing frequency poses a challenge when it comes to achieving the desired therapeutic outcomes. This was seen in a combined analysis comparing the efficacy of pegcetacoplan and avacincaptad pegol, which revealed differences in therapy results concerning dosing and frequency.80 Here, EOM administration of pegcetacoplan demonstrated comparable effectiveness with monthly administration of avacincaptad pegol in slowing the progression of GA, but monthly administration of pegcetacoplan exhibited greater effectiveness (p<0.05) in reducing the lesion growth than monthly avacincaptad pegol. The burden of treatment frequency from monthly and EOM injections remains a challenge in a real-world setting as many patients with GA secondary to AMD are elderly with financial and mobility-related burdens. The acceptability of long-term intravitreal complement inhibitor therapy by the patient population with GA has yet to be quantified, but results from a moderately sized cross-sectional study in the UK addressing this question are pending.81
Up to this point, complement inhibitors do not demonstrate a complete cessation of lesion growth. However, noteworthy insights from the GATHER and GALE trials suggest that the continued divergence in lesion growth curves with time could closely achieve a plateauing characteristic with long-term treatment. The concern for CNV remains at large, with no clear explanation behind the neovascular conversion in eyes lacking it at baseline. Molecular investigations suggest that complement inhibition of C3 decreases the clearing of astrocytes by microglia and thus may increase the astrocyte accumulation in retinal cells with depleted C3, which consequently increases the expression of extracellular matrix structures and integrins involved in angiogenesis.82–84 This convolutes with treatment frequency, as monthly treatments demonstrate a slower progression of GA but are concurrently associated with an elevated occurrence of eAMD compared with EOM treatments. This could be solved by administering anti-angiogenic agents as adjuncts with complement inhibitors to decrease the risk of CNV conversion.
Overall, the development of effective and safe complement inhibitors for GA secondary to AMD is still in its nascent stages with several challenges as discussed in this section, but the outlook remains hopeful for the development of a complement inhibitor therapy regimen of reasonable frequency that leads to the cessation of growth of atrophic lesions and improvement in visual metrics. Potential adjunct therapy with anti-VEGF to counter the appreciable risk of eAMD in eyes undergoing treatment may be necessary. Other medical avenues for GA being tested include the categories of gene therapy (JNJ-1887), stem cell therapy (e.g. RG6501 and iPSC-derived RPE/PLGA (polylactic-co-glycolic acid) transplantation), neuroprotective agents (e.g. brimonidine) and others (e.g. BI 771716 and CT1812).85–90
Conclusion
Recent advancements in understanding the pathogenesis of GA have underscored the crucial role of the complement system, leading to the development of novel therapeutic approaches targeting this pathway. The approval of pegcetacoplan and avacincaptad pegol by the FDA marks a significant milestone in GA management, offering new hope for patients by effectively slowing the progression of retinal atrophy. Pegcetacoplan and avacincaptad pegol, through their distinct mechanisms of inhibiting complement factors C3 and C5, respectively, have demonstrated efficacy in blunting atrophic lesion growth in clinical trials. These therapies have also highlighted the importance of continued exploration and optimization of complement inhibitors, with ongoing trials investigating additional agents targeting various components of the complement pathway. The promising results from current trials suggest a new landscape of AMD and retinal degeneration treatment.