Small pupils present a significant challenge for the cataract surgeon, requiring excellent intraoperative decision making to maximize the likelihood of a successful outcome. One of the common causes of small pupils, intraoperative floppy iris syndrome (IFIS), in particular increases the likelihood of complications such as posterior capsule rupture and iris trauma. Fortunately, a variety of pharmacologic and mechanical strategies are available to help maintain an adequately dilated pupil to increase the likelihood of successful, safe cataract surgery.
IFIS was first described in association with current or prior tamsulosin (Flomax®, Boehringer Ingelheim, Ingelheim am Rhein, Germany; Ridgefield, CT, US) use in 2005.1 Besides poor preoperative pupil dilation, severe IFIS exhibits a triad of intraoperative signs: (1) iris billowing and floppiness, (2) iris prolapse to the incisions, and (3) progressive intraoperative miosis (Figure 1). However, there is a wide range of clinical severity. IFIS can be graded as mild (good dilation; some iris billowing without prolapse or constriction), moderate (iris billowing with some constriction of a moderately dilated pupil), or severe.2 In a prospective study of 167 eyes in patients taking tamsulosin, 10% had no IFIS, 17% mild IFIS, 30% moderate IFIS, and 43% severe IFIS.2 Poor preoperative pupil dilation and iris billowing during instillation of intracameral lidocaine are predictive of greater IFIS severity.2–5 Numerous studies have confirmed that unexpected IFIS increases the rate of cataract surgical complications.2,3,6–9 A retrospective study of nearly 100,000 surgeries in male patients with cataracts reported a two-fold increase in the rate of serious postoperative complications including retinal detachment, retained nuclear fragments, and severe inflammation in patients taking tamsulosin.6
IFIS has since been documented with other systemic alpha-1 antagonists such as doxazosin (Cardura®, Pfizer, New York, NY, US), terazosin (Hytrin®, Abbott, Chicago, IL, US), and alfuzosin (Uroxatral®, Sanofi Aventis, Paris, France), all of which are non-selective alpha-1 antagonists.3 A number of retrospective and prospective studies have also shown that the frequency and severity of IFIS is much higher with tamsulosin than non-selective alpha-1 antagonists.3,4,6–8,10,11 Silodosin (Rapaflo®, Allergan, Irvine, CA, US) is a systemic selective alpha-1A blocker that is similar to tamsulosin in its strong propensity to cause IFIS. Ophthalmologists should also know that Jalyn® (GlaxoSmithKline, Brentford, UK) is the brand name for the combination of dutasteride and tamsulosin.
The surgeon should assess the quality of dilation during the presurgical examination, but a careful history is also important to reduce the likelihood of unexpected intraoperative miosis. In fact, even female patients are now treated with tamsulosin for urinary problems.12
Unfortunately, stopping tamsulosin preoperatively is of questionable value.2 Preoperative counseling with high-risk patients should occur to discuss the risk of IFIS and subsequent iris and pupil changes.
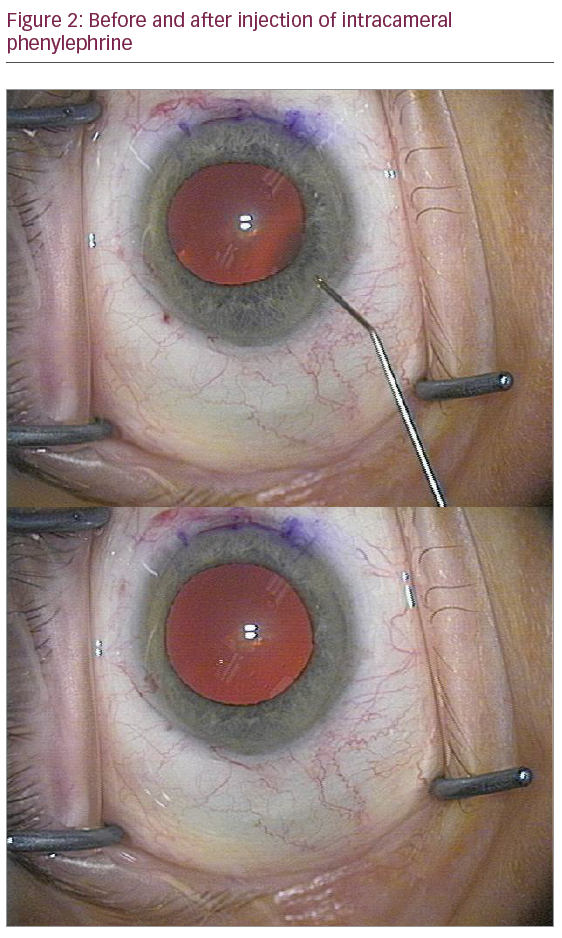
Intracameral injection of alpha agonists such as phenylephrine or epinephrine, as first reported by Gurbaxani & Packard and Shugar, respectively, is a safe and inexpensive strategy for IFIS (Figure 2).13–15 Healon 5® (2.3% sodium hyaluronate; Abbott Medical Optics, Santa Ana, CA, US) is a maximally cohesive single agent that is particularly well suited for viscomydriasis and for blocking the iris from prolapsing in IFIS, as first suggested by Osher & Koch.16 In the tamsulosin versus alfusosin study, moderate to severe IFIS was present in 12.4% of control eyes performed without epinephrine in the irrigating fluid, demonstrating for the first time the beneficial impact of having epinephrine in the irrigating fluid during phaco.5

Pupil expansion rings are disposable devices that mechanically expand and maintain the intraoperative pupil diameter. The semicircular Morcher® 5S Pupil Dilator (FCI Ophthalmics, Pembroke, MA, US) and the Perfect Pupil® (Milvella, Savage, MN) are grooved polymethyl methacrylate (PMMA) rings.17,18 Alternatives include the APX 200 (FCI Ophthalmics, Pembroke, MA, US) and Graether pupil expander (Eagle Vision, Memphis, TN, US).19 All of these devices are relatively difficult to position if the pupil is less than 4 mm wide or if the anterior chamber is shallow. They will fail to engage the iris if the pupil diameter is larger than 7 mm.
The Malyugin Ring® (MicroSurgical Technology, Redmond, WA, US) is a 5–0 polypropylene single use device that is introduced with a disposable injector (Figure 3).20 The way in which the iris drapes over the sides of the device creates a round 6.25 mm or 7 mm pupil diameter. Compared to the bulkier plastic expansion rings the thinner profile of the Malyugin Ring reduces the risk of accidental corneal or incisional trauma and does not impede instrument access to the cataract. The Malyugin Ring may be preferred to iris hooks in the presence of a bleb, a pterygium, keratoconus, and radial keratotomy scars, and avoids the problem of iris hooks being pushed against the lid speculum with a tight palpebral fissure or uncooperative patient. The OASIS Iris Expander (OASIS Medical, San Dimas, CA, US) is a similar polypropylene device with four pockets instead of rings. Neither device can engage pupils dilated beyond 7 mm, however.
Iris retractors provide sufficient tension to the iris stroma so that no prolapse can occur with IFIS (Figure 4). Maximal widening with iris retractors can be safely employed with IFIS eyes because of the elasticity of the pupil margin. Iris retractors are advantageous when a toric intraocular lens (IOL) is used because two of the retractors can often be aligned with the astigmatic axis. A particular advantage of using iris retractors for cases with weak zonules is that the retractors can be readily repositioned around the capsulorrhexis edge to serve as capsule retractors if necessary. Reusable 4–0 polypropylene retractors (available from Katena Products, Inc., Denville, NJ, US and FCI Ophthalmics) are much more cost effective than any other pupil expander, including disposable 6–0 nylon retractors (available from Alcon Laboratories, Inc., Fort Worth, TX, US).21 The greater rigidity makes 4–0 retractors more durable and easier to manipulate.


Before initiating the capsulorrhexis, 1 mm limbal paracenteses are created in each quadrant, including a separate stab incision just posterior to the temporal clear corneal incision in a diamond configuration as originally advocated by Oetting and Omphroy.22 Alternatively, a 25 or 26 gauge needle can be used to make tracts for the hooks.22,23
Use of tamsulosin continues to increase in the cataract population, but ophthalmologists can continue to deliver great results thanks to awareness of each patient’s risk for IFIS and familiarity with a few of these surgical management techniques.













